.png?width=290&height=155&name=kemri_0%20(1).png)
KEMRI Cuts Approval Time by 60% with eMudhra’s emSigner Gateway

About The Customer
The Kenya Medical Research Institute (KEMRI) is Kenya’s premier public-health research organization, conducting critical studies in epidemiology, clinical trials, and public-health interventions.
Impact at Glance
Business Scenario
KEMRI had automated many workflows in Dynamics ERP—but lacked a native, legally binding digital-signature capability. As a result:
- Manual sign-off processes delayed approvals by 3–5 days
- Scanned/PDF signatures carried non-compliance risk under Kenyan e-signature laws
- No centralized, tamper-proof audit trail for critical document
eMudhra Solution
emSigner Gateway: an API-driven middleware that plugs into Microsoft Dynamics to deliver legally compliant, automated digital signatures without changing end-user workflows.
Key Features of the Solution:

Legally Compliant Signatures: Direct integration with Kenya’s Root CA per the Electronic Certification Regulations

API-Based Integration: Zero-code impact on existing Dynamics forms and approvals

Full Audit Trails: Timestamped, non-repudiable logs for each signature event

Automation-Ready: Bulk signing and workflow-triggered approvals across departments
Solution Architecture
emSigner Gateway sits between Dynamics ERP and Kenya’s Root CA, using cloud HSMs and TLS-encrypted APIs for secure, scalable signing. Its containerized, stateless design and centralized logging ensure high availability and real-time audit visibility. Below shared is the implementation process for the same.
Implementation Process

Rapid Deployment
emSigner Gateway configured and live within KEMRI’s Dynamics sandbox in under two weeks.

Administrator Training
Hands-on workshops for IT & compliance teams on managing signature policies and audit reports.

End-User Onboarding & Go-Live Support
Step-by-step user guides, Q&A sessions, and dedicated hypercare during the first approval cycles.
Architecture Diagram

Value Added to Kenya Medical Research Institute
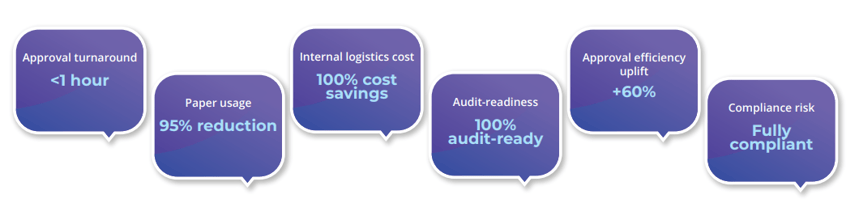
Sub-hour Approvals:
Critical documents signed & routed in under 60 minutes
95% Paper Savings:
Sharp reduction in printing, storage, and physical handling
Zero Logistics Cost:
Eliminated inter-office courier and manual delivery expenses
60% Efficiency Uplift:
Faster decision-making across procurement, finance & HR
100% Audit-Readiness:
Instant generation of compliance reports for regulators
![]()
Risk Mitigation:
Fully compliant, non-repudiable signatures under Kenyan law
Conclusion
By embedding eMudhra’s emSigner Gateway into Microsoft Dynamics, KEMRI transformed a slow, manual approval cycle into a secure, fully digital process—driving massive efficiency gains, eliminating compliance risks, and supporting Kenya’s broader digital-signature mandate.
